Soil Science Fundamentals
By Jake Eiting, Horticulturist
To help plants thrive and to maximize your time and resources invested in a garden, you’ll need to get to know your soil. An inexpensive soil test will give you valuable information about your soil texture, mineral content, and chemistry.
How does this help your garden? Soil texture and the aggregates it forms affect water infiltration and retention. Mineral content tells us what nutrients are in the soil or are deficient. Soil chemistry gives us information about a nutrient’s availability or unavailability even though it may be present in the soil. Taken together, these factors give us vital information such as how to water, fertilize, and diagnose plant nutrient deficiencies.
Soil texture
Typically, a soil’s composition is as follows: 25% pore space (air), 25% water, and 50% particle matter. Of the 50% particle matter, up to 10% could be organic matter. Organic matter, or humus, is the rich, dark bits of decomposed plant materials or animal wastes. Soils in climates with high precipitation and dense plant cover have greater organic matter content, but in Utah’s dry climate with lower plant density, our organic matter content is between .25% to 1%. The remaining 90% or more of soil particle matter is inorganic, often in the form of sand, silt, and clay. Sand is the largest of these three particles, followed by silt, and then clay. Clay particles are so small that they’re not visible by ordinary light microscopy.
Learn about Soil Science Fundamentals.
Image 1: Chart showing soil composition. Credit: Jake Eiting
Image 2: Soil particle size to scale. Credit: Jake Eiting
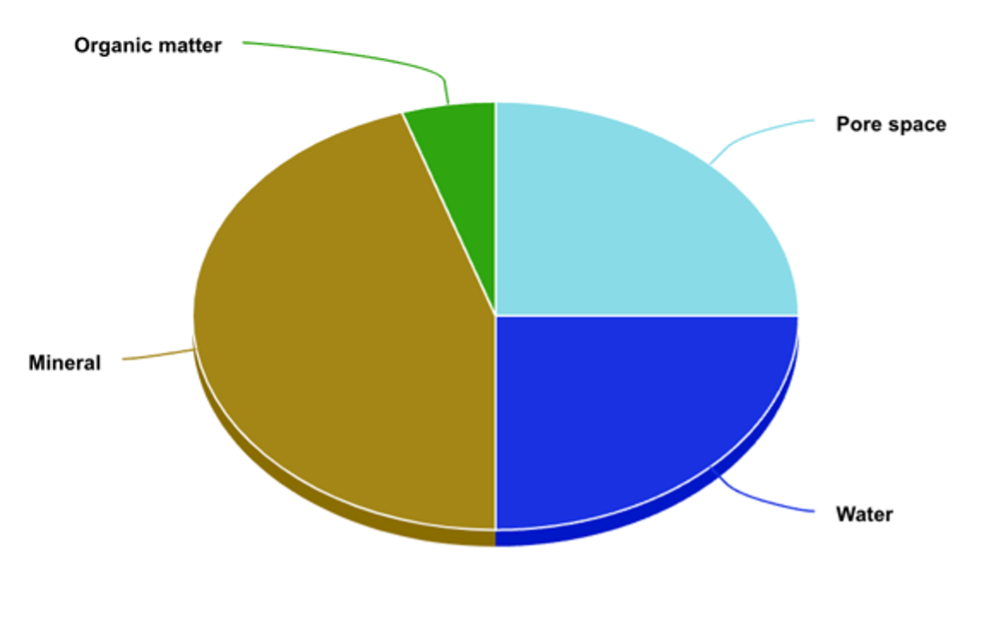
Figure 1

Figure 2
Soil texture, or when a soil is described as sandy, silty or clay, refers to the relative abundance of those specific particles. For example, a soil classified as “sandy” has 70% or more of sand particles, “clay” has 40% or more clay particles, and “silt” has 80% or more of silt particles. A loam refers to a soil having a somewhat equal mixture of three particle types. The relative combinations of these particles create an abundance of soil textures such as silty loam, sandy clay, and others. The National Resources Conservation Service (NRCS) soil textural triangle can help you learn more about your soil’s textural class.
Soil texture determines how quickly water moves through the soil and how easily plant roots can take uptake that water. Sand particles are large and cannot sit very closely to one another. This creates large pore spaces between particles where water can easily infiltrate and drain from, and also allows roots to access more oxygen. Clay particles are much, much smaller and can sit extremely close to one another. This means very small pore spaces but more pore space overall; consequently, clay soils absorb more water and release it very slowly. Silt’s size and water holding characteristics lie in between sand and clay.
For plants growing in a sandy soil, roots will have an easier time taking up water; however, roots will only be able to absorb some of this water before it drains beyond reach. The inverse is true for plant roots growing in clay soil. Roots will have a harder time taking up water held tightly by clay particles, but the water will be available for longer due to the slow-draining nature of clay soils.
Soil aggregates refer to particles of sand, silt, clay and organic matter bound together in the soil. These aggregates vary in size and shape and are generally a good indicator of soil health. They facilitate water infiltration and drainage and provide habitat for beneficial soil microorganisms, while soils with poor aggregation tend to break down faster and are less productive.
Aggregates form in a complex process where compounds secreted from soil fauna and plant roots bind together the various particles present in a soil. Testing is available to help determine a soil’s aggregate stability.
Soil chemistry
Texture also influences soil’s nutrient availability. In soil science, nutrient availability is often approximated using a term called cation exchange capacity (CEC). CEC measures a soil’s ability to absorb positively charged minerals, known as cations. Many nutrients that plants require are cations, such as potassium (K+), calcium (Ca2+), and magnesium (Mg2+).
Clay and organic matter particles have many more negatively charged “sites” in which a cation can attach. That gives clay and organic matter a much higher CEC compared to pure sand which has essentially no cation exchange capacity.
One positively charged particle that has as strong impact on soil chemistry is the hydrogen ion, or H+. The concentration of hydrogen ions within a substance determines its pH. pH is measured in units, on a scale of 0 to 14, with the value of 7 considered neutral. Values above 7 are basic or alkaline, and values below 7 are considered acidic.
Soils exhibit various pH values based on many factors such as organic matter content, parent material, and degree of weathering. Paying attention to a soil’s pH value is important since certain soil nutrients can become more or less available to plants at varying levels of acidity or alkalinity. For example, our soils along the Wasatch Front tend to be slightly alkaline (7.5–8) on average. In these conditions, minerals that are present in the soil, such as Iron (Fe), Manganese (Mn), and Zinc (Zn), can be difficult for plants to uptake and can result in plant disorders like iron chlorosis. In moderately acidic conditions (5.5-6) Calcium (Ca) and Phosphorus (P) can be similarly difficult for plants to uptake.
Soil minerals
Plants require fifteen key nutrients to perform a plethora of functions including metabolic duties, photosynthesis, respiration, protein synthesis, and volatile compound production. Three of these nutrients (hydrogen, carbon, and oxygen) are obtained from the atmosphere, the other twelve are obtained from the soil. Those required by plants in the largest amounts are referred to as macronutrients and include nitrogen, phosphorous, potassium, calcium, magnesium, and sulfur. Nutrients required in lower amounts, or micronutrients, include iron, manganese, copper, zinc, boron, and molybdenum.
Plant performance follows the “law of minimum” which means that the most deficient nutrient will be the limiting factor even when all other nutrients are readily available. A soil test will tell you what nutrients are available in your soil and will help you determine what steps to take to optimize performance.
Testing your soil
Several tests can be conducted to learn about the various aspects of a soil. You can easily perform a few tests at home but soil laboratories provide a higher level of confidence. In either case, the soil collected for a test should be a uniform sample from various locations within your garden or yard with the same or similar soil type. If your property contains more than one soil type, then you’ll need additional soil tests.
- Soil pH is easily measured at home with at-home soil test kits, a digital pH meter or probe, or strips designed for testing soils.
- Soil texture can be ascertained at home as well but requires practice and a slight familiarity with soils. To learn the texture of a soil without laboratory equipment, you can use a method known as texture-by-feel, which requires only your hands and a small amount of water.
- Other elements usually calculated in a laboratory setting are nutrient percentages, nutrient deficiencies, and organic matter percentage.
Utah State University offers inexpensive soil testing for the public. For more information about how to proceed with the results of your soil test, check out our articles on fertilization, iron chlorosis, and soil improvement.
Soil science can seem intimidating at first but learning just a few basics will help you interpret your soil test results and make better choices about how to manage your soils and help your plants thrive.
Learn how to Manage Your Soils.